Zinc-air batteries are representatives of a group of metal-air systems in which electricity is generated by the electrochemical pair of metal (electrosurgical metal) and the oxygen in the air, i.e. the presence of only one electrode apriori makes system cheaper and lighter.
Primary zinc-air batteries have found a number of commercial applications, as well as the mechanically rechargeable ones. The challenge for science and industry is the development of electric rechargeable zinc-air batteries. Regardless the intensive research from the beginning of the 21st century, there are still many problems to be solved.
The main barrier towards commercialization of zinc-air batteries is the lifetime (number of discharge / charge cycles), which is related to the degradation of the electrodes during cycling and leads to a rapid decrease of their capacity. Despite the numerous studies, it is not still solved.
The working principle of the Zinc-air battery is shown on the figure bellow. During discharge the metal electrode (anode) is oxidizing with oxygen from the air, which is reduced at the air - gas-diffusion electrode (cathode), creating an electromotive force. The presence of only one electrode apriori makes possible the achievement of high energy density (1.3 kWh / kg-1). During charge regime the opposite reaction with realease of oxygen is performed.
| CATHODE Gas-diffusion electrode |
ANODE Zinc electrode |
|
|---|---|---|
| Discharge: | О2 + 2H2O + 4е → 4ОН ORR (рoxygen reduction reaction) |
Zn + 4OH- → [Zn (OH)4]2- [Zn (OH)4]2- → ZnO + H2O + 2OH- |
| Overall reaction: 2Zn + O2 → 2ZnO | ||
| Charge: | 4OH- +O2 → 2Н2O + 4е- OER (oxygen evaluation reaction) |
ZnO + H2O + 2OH- → [Zn (OH)4]2- [Zn (OH)4]2- + 2е- → Zn + 4OH- |
| Overall reaction: 2ZnO → 2Zn + O2 | ||
Components in Zn Air Secondary Batteries
Junghye Lee and Ketack Kim, Journal of the Korean Electrochemical Society, Vol. 16, No. 1, 2013, 9-18
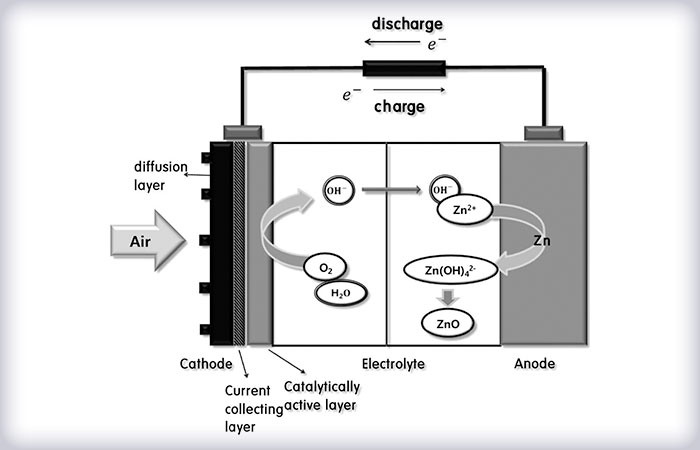
The Gas-Diffution Electrode (GDE) consists of two layers - a porous gas-diffusion layer GDL, which serves for oxygen transport, and a catalytic (active) layer (CL), which ensures the electrochemical reaction (reduction of oxygen during charge and oxidation, i. e. releasing oxygen during charge), in combination with a metallic collector.
The electrochemical reaction occurs at the triple-phase (TRB) electrode / electrolyte / gas phase.
Disadvantages: The main problem associated with gas-diffusion electrode is the high electrochemical carbon corrosion at charging, which reduces the stability of the electrode.
The Zink electrode: From the group of metals used Mg, Zn, Al, Fe, Ca, Li, zinc is the most active one in aqueous electrolytes, also it is cheaper and non-toxic, it operates in humid environment and water electrolytes, and has low self-discharge. It can be recharged via ZnO, the cell construction is simple, the system has a long calendar life and a “flat “ discharge curve.
Disadvantages: The main problem associated with the zinc electrode, some of them must overcoming, some of them adapted to the area of applicability, are (1) corrosion (release of hydrogen as a parasitic reaction) and dissolution, (2) dendritic formation at charge, combined with electrode change due to nonuniform dissolution / of zinc; (3) passivation of zinc electrode with ZnO layer formation due to zinc ions saturation (i ZnO - reversible process and (i)i ZnO - irreversible process).
These negatives of zinc-air batteries, originating from the zinc electrode, can be successfully used in integrated energy storage systems, for example in combination with lead-acid batteries, lithium-ion or metal hydride etc.
The Electrolyte: For rechargeable batteries, alkaline electrolytes based on KOH (6-7 M) are the most common electrolyte, as it has a maximum ionic conductivity (640 mScm-1) at 25oC temperature, which determines the rapid kinetics of the oxygen reduction reaction. Intensive work on "near-neutral electrolytes", non-aqueous electrolytes, solid phase (polymer) electrolytes is also running.
Disadvantages: zinc corrosion (dissolution of zinc salts or hydroxides); precipitation of insoluble carbonates; electrolyte evaporation; hydrogen evolution.